Vanadium Redox Flow Batteries
The vanadium redox flow battery (VRFB) is the simplest and most developed flow battery in mass commercial operations.
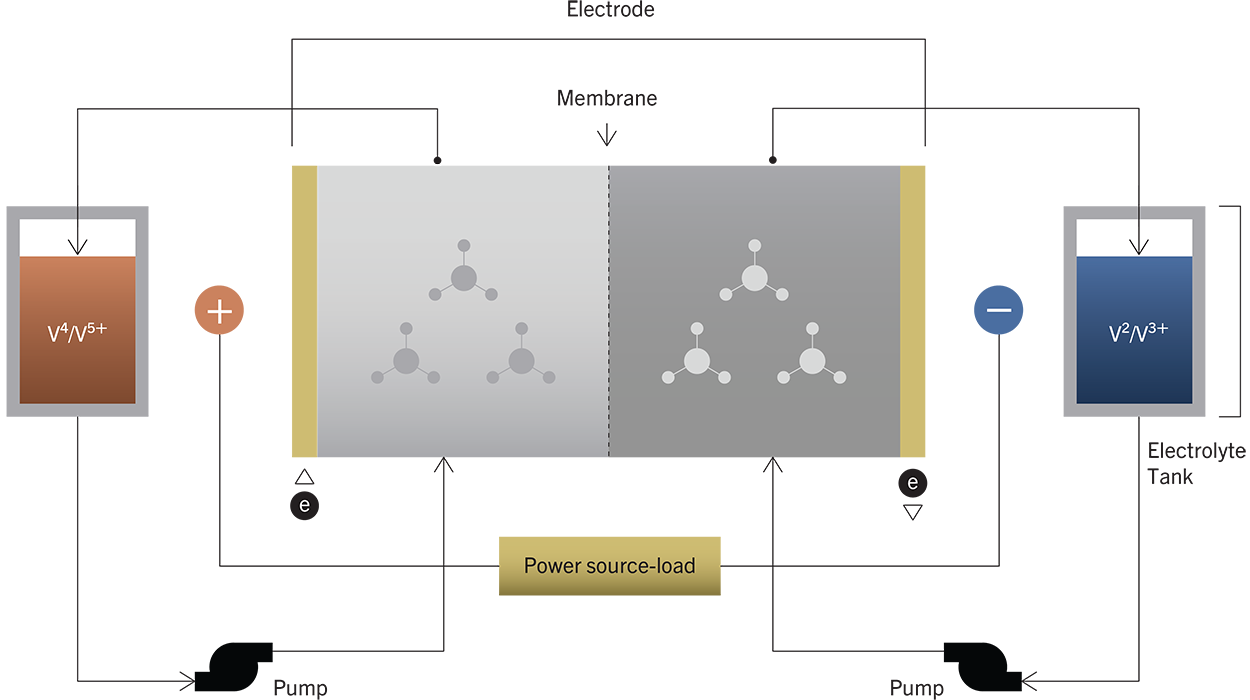
It was first developed by the National Aeronautics and Space Administration (NASA) in the 1970s. Unlike conventional batteries, the liquid electrolytes are stored in separated storage tanks, not in the power cell of the battery.
During operation, these electrolytes are pumped through a stack of power cells or membranes, where a reversible oxidation (redox) electrochemical reaction takes place – charging or discharging the battery.
Vanadium can exist in four different states, allowing for a single element to be used to store energy. Vanadium was first used in flow batteries in the mid-1980s.
In addition to vanadium, the electrolyte consists primarily of water and chemical additive acids, such as sulphuric acid or hydrochloric acid.
Vanadium redox flow batteries are well-positioned to take a significant share of the stationary energy storage market, owing to unique advantages for long-duration stationary energy storage applications.
Energy Storage Solution
LONG LIFESPAN CYCLES
Ability to repeatedly charge / discharge more than 35 000 times for a lifespan of over 20 years
100% DEPTH OF DISCHARGE
Discharge without material performance degradation is unique to VRFBs
LOW COST
Low cost per kWh when fully used at least once daily (cheaper than Li-ion batteries)
SPEED
Rapid response time of less than 70 ms
FLEXIBILITY
Flexibility that allows for capturing the multi-stacked values of energy storage in grid applications
SUSTAINABILITY
30% lower carbon footprint than li-ion batteries – 100% of vanadium is re-usable on decommissioning of a system
NO CROSS-CONTAMINATION
No cross-contamination since there is only one battery element – unique among flow batteries
SAFETY
Safe operation with no risk of fire or smoke from thermal runaway
